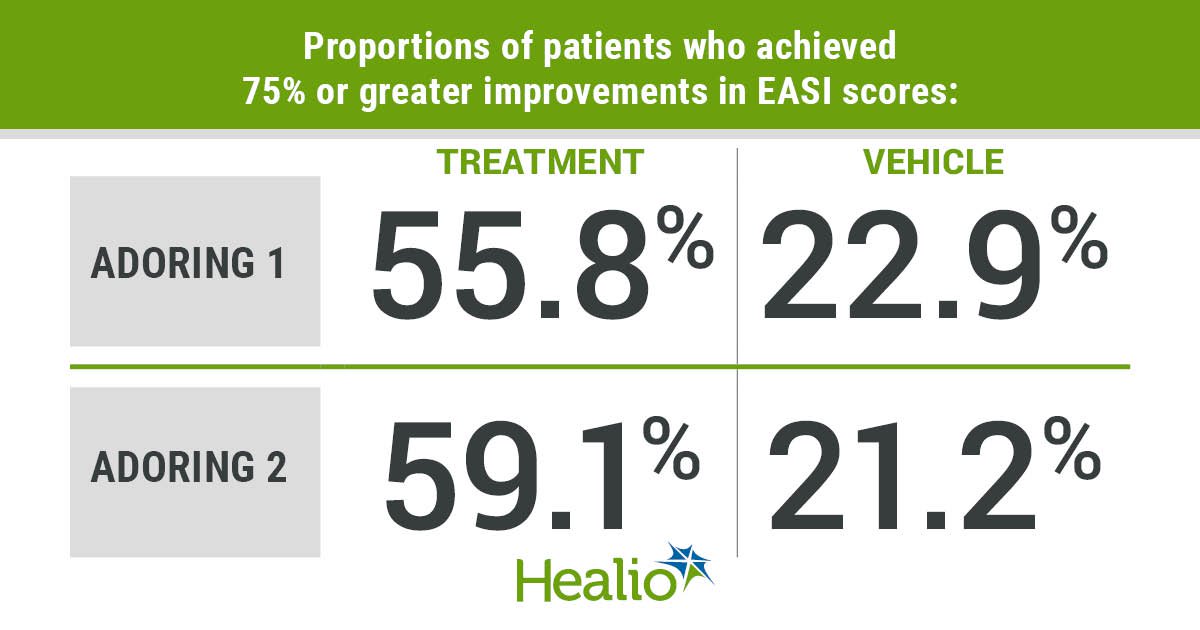
Tapinarof, a steroid-free topical treatment targeting atopic dermatitis, showed significant improvement in symptoms, especially itch, for patients in a clinical trial presented at the American Academy of Allergy, Asthma & Immunology Annual Meeting. The cream, named Vtama, has been submitted for FDA approval for patients aged 2 years and older. The treatment is considered suitable for sensitive areas of the skin and offers long-term relief. Results from the phase 3 trials showed statistically significant improvements in disease severity and age subgroups, with mild to moderate adverse events reported. Further studies are being conducted to explore the long-term effects of tapinarof cream.
Source link
Daily tapinarof use leads to 75% improvements in Eczema Area and Severity Index scores