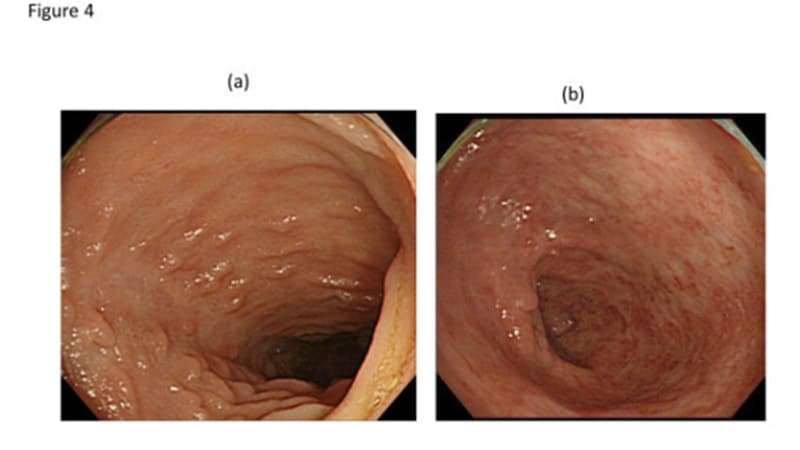
Long-term data on the use of risankizumab in moderate to severe Crohn’s disease show stable rates of clinical remission and endoscopic response for up to 3 years, according to the FORTIFY extension study. Patients on risankizumab showed less antibody formation compared to other treatments, and the drug’s efficacy was consistent whether used as first-line treatment or after failing other compounds. Risankizumab’s safety profile was favorable, with manageable adverse events. The results suggest that risankizumab provides an effective and durable treatment option for some patients with Crohn’s disease and offers a favorable benefit-risk ratio.
Source link
Outcomes Remain Stable up to 3 Years