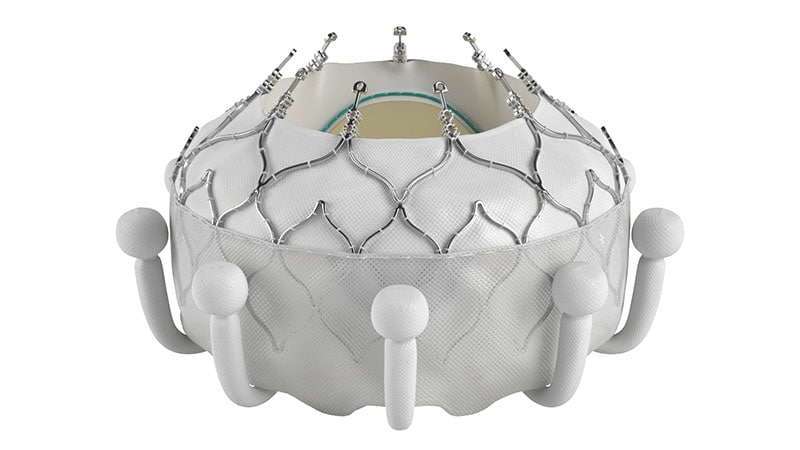
The FDA has approved Edwards Lifesciences’ Evoque tricuspid valve replacement system, the first transcatheter therapy for tricuspid regurgitation in the United States. It is indicated for improving health status in patients with severe tricuspid regurgitation despite optimal medical therapy, offering a less risky option for older patients. The system, which includes a self-expanding frame, intra-annular sealing skirt, and tissue leaflets, has shown to be superior to medical therapy alone in a study. Results include significant reduction or elimination of tricuspid regurgitation and sustained quality of life improvement. The Evoque system received CE Mark approval in Europe in October 2023 and is expected to be presented at the TCT meeting in 2024.
Source link
FDA OKs First Transcatheter Tricuspid Valve Replacement