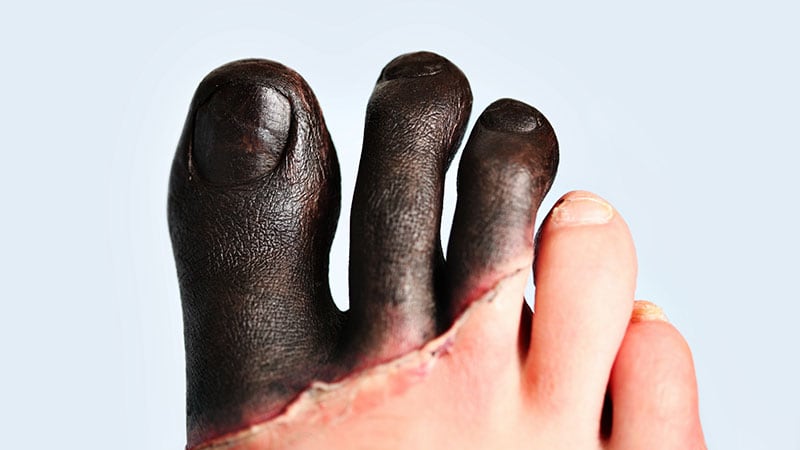
The FDA has approved iloprost injection for treating severe frostbite in adults to reduce the risk of amputation. A randomized trial showed that iloprost alone had a 0% risk of amputation based on bone scans, compared to 60% with buflomedil on day 7. The most common adverse events with iloprost are headache, flushing, heart palpitations, fast heart rate, nausea, vomiting, dizziness, and hypotension. Iloprost is the first-ever treatment approved for severe frostbite, providing physicians with a tool to prevent amputation of frostbitten fingers or toes. This approval represents a significant advance in the treatment of severe frostbite.
Source link
FDA Approves Iloprost for Severe Frostbite