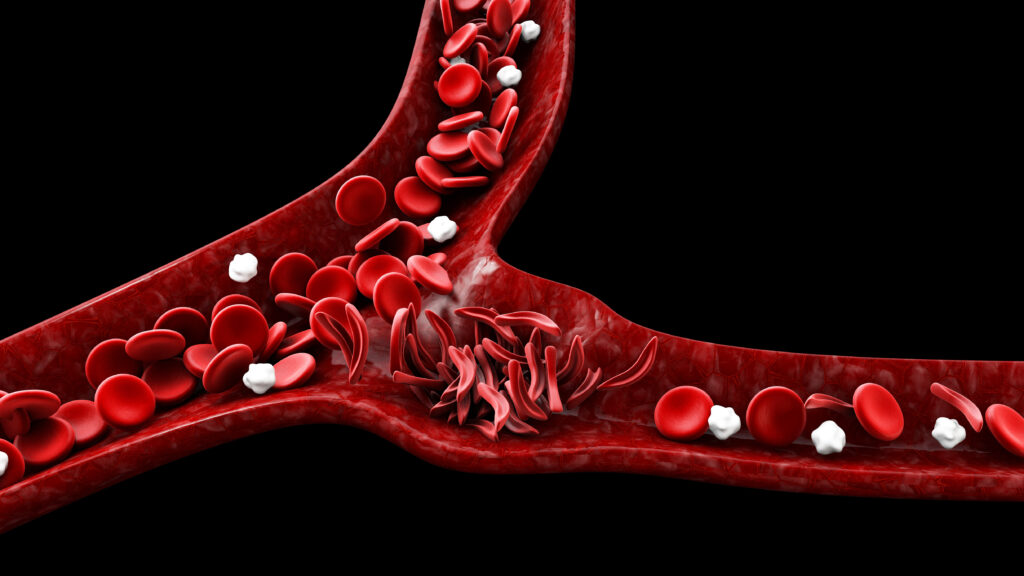
The Food and Drug Administration approved the first gene therapies for sickle cell disease. The approval sparked discussions at the American Society of Hematology meeting, where doctors and researchers expressed measured optimism about the landmark shift in the treatment of the disease. However, questions were raised about whether these innovative treatments will reach the patients who need them most, due to issues like cost, limited treatment centers, and patient and provider education. Despite skepticism, some physicians see it as groundbreaking and believe it will transform care for sickle cell patients. The event gave hope for the future but also raised concerns about accessibility and implementation of these advanced treatments.
Source link
Experts at ASH cautiously optimistic about sickle cell gene therapies