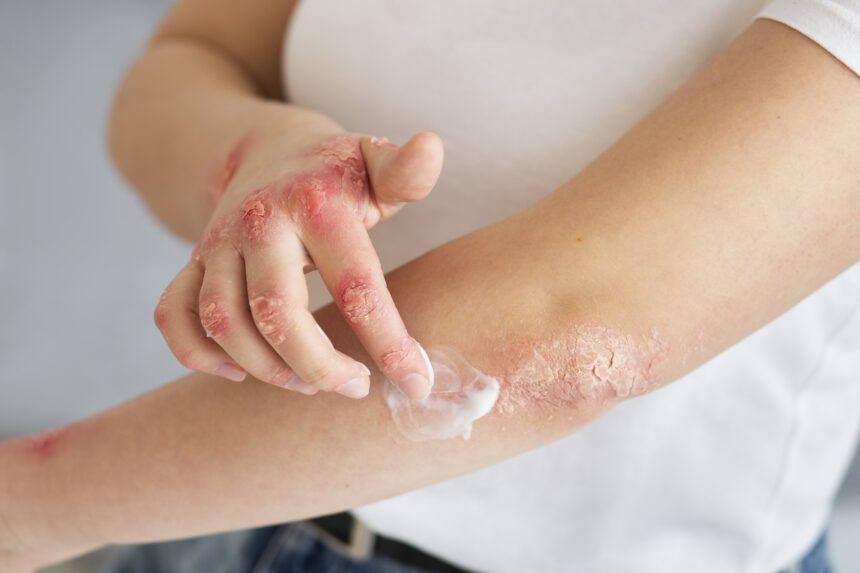
The U.S. FDA has approved Eli Lilly’s new eczema drug, Lebrikizumab branded as Ebglyss, for moderate-to-severe atopic dermatitis. Atopic dermatitis is a common skin disease that causes dry, itchy patches. The drug showed significant improvement in clinical trials, providing relief in as little as two weeks. It is recommended for adults and children over 12 who weigh at least 88 pounds. The initial treatment involves injections every two weeks, followed by maintenance doses every four weeks. The drug offers hope for the millions of Americans suffering from eczema, providing a new treatment option when topical prescriptions are not enough.
Source link
Eli Lilly’s Eczema Drug Gets FDA Approval, Injectable Treatment Available Within Weeks