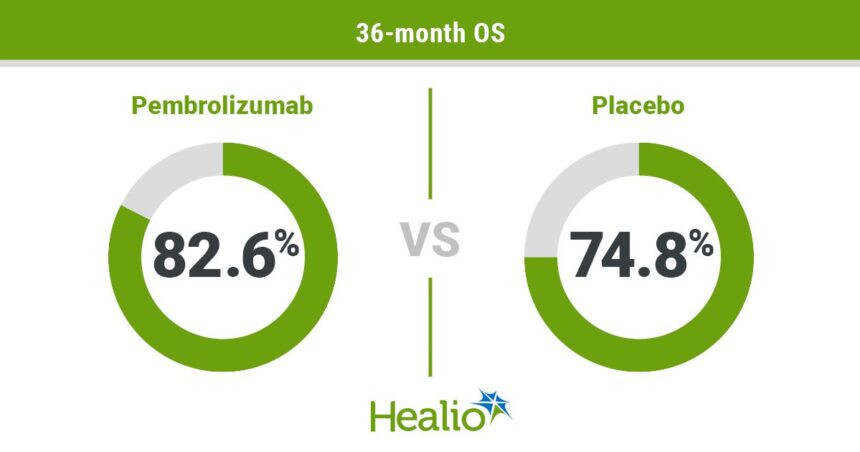
Pembrolizumab plus chemoradiotherapy has shown to significantly improve overall survival for women with high-risk locally advanced cervical cancer. The FDA approved this regimen based on progression-free survival data from a phase 3 trial. Results presented at the ESMO Congress demonstrated a higher 36-month OS rate with pembrolizumab compared to a placebo. The regimen showed consistent benefits across all subgroups and had a manageable safety profile. This treatment combination should be considered the new standard of care for women with high-risk locally advanced cervical cancer. The study was funded by Merck Sharp & Dohme, and the lead researcher has financial relationships with various pharmaceutical companies.
Source link
Data support pembrolizumab regimen as ‘new standard’ for advanced cervical cancer