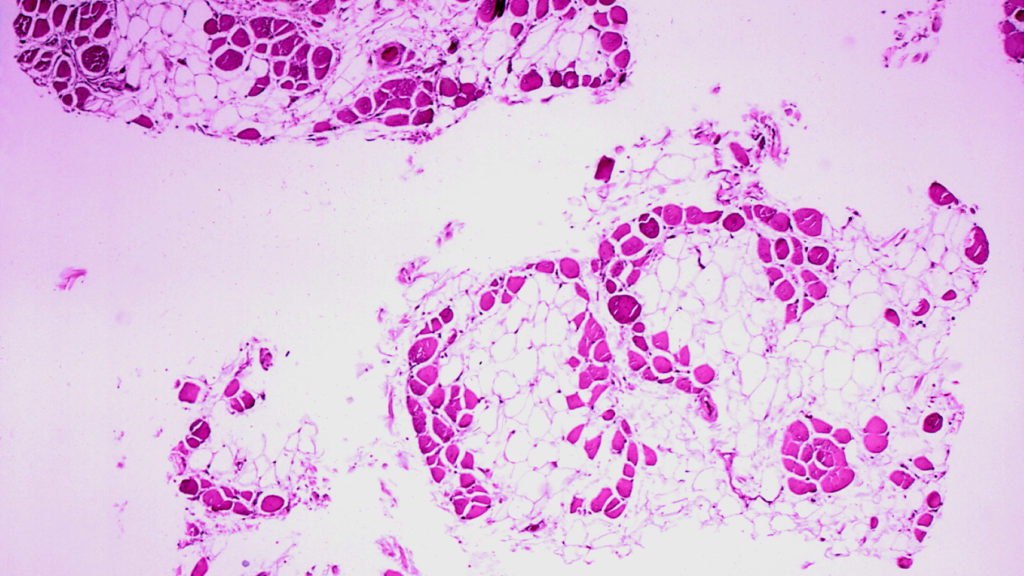
Japanese drugmaker Nippon Shinyaku announced that its Duchenne muscular dystrophy drug, Viltepso, failed to meet its primary endpoint in a placebo-controlled confirmatory trial. The drug, part of a class of treatments for the rare disease called exon-skippers, did not show statistically significant differences compared to the placebo in terms of improving muscle function. Although children receiving Viltepso were able to stand up faster, the overall clinical benefits of exon-skippers have not been proven in large studies. This failure is significant as it challenges the efficacy of drugs designed to increase dystrophin protein levels in patients with specific mutations.
Source link
Duchenne drug from Nippon Shinyaku fails in rare confirmatory trial