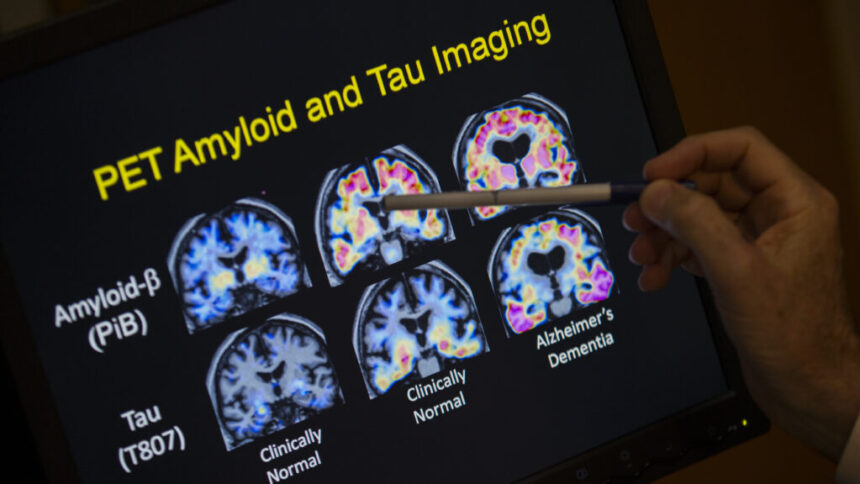
The FDA approved lecanemab as an anti-amyloid drug for Alzheimer’s patients, but its safety for Down syndrome patients is unknown due to higher risk factors for brain hemorrhages and swelling. Research suggests caution in prescribing these drugs to this population. Limited data on Alzheimer’s in Down syndrome highlights the need for more inclusive clinical trials. Treatments like lecanemab could increase lifespan and reduce caregiving costs, yet research has excluded individuals with Down syndrome. Future trials plan to address this gap, but caution is advised due to potential side effects in this population. The scientific community is urged to prioritize safety in prescribing anti-amyloid drugs like lecanemab to individuals with Down syndrome.
Source link
Leqembi and the risk of brain bleed in patients with Down syndrome