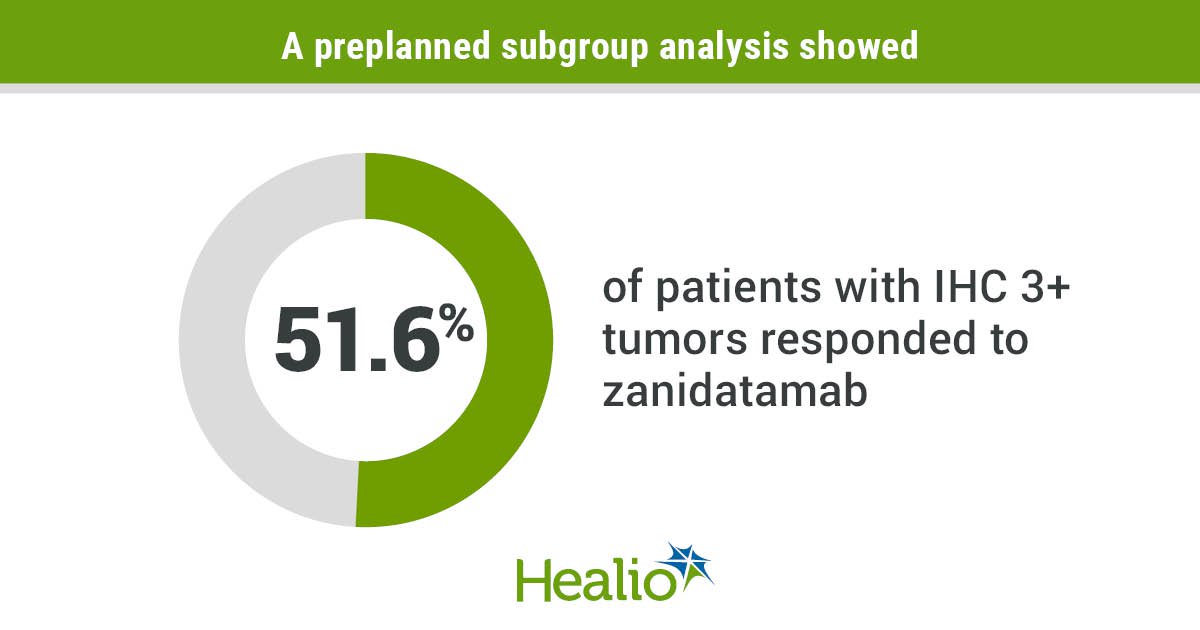
Results of the phase 2B HERIZON-BTC-01 study showed that zanidatamab exhibited durable antitumor activity in patients with previously treated advanced HER2-amplified biliary tract cancer. More than half of the patients who received zanidatamab monotherapy survived at least a year, with a median overall survival that exceeded 15 months. The study included 87 participants with various types of biliary tract cancer, and the results were presented by researcher Shubham Pant at the ASCO Annual Meeting. Safety analyses showed that most patients experienced treatment-related adverse events, but they were easily managed. Jazz Pharmaceuticals is seeking accelerated approval for zanidatamab for this indication.
Source link
Zanidatamab exhibits ‘promising’ efficacy in advanced HER2-positive biliary tract cancer