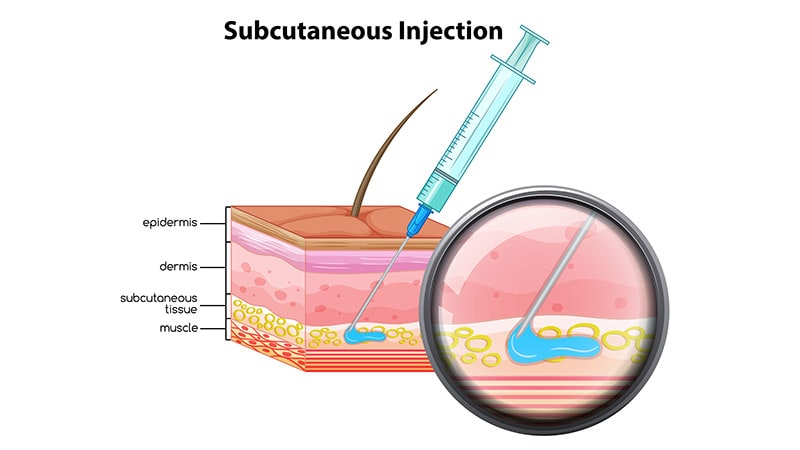
Administering the bispecific antibody amivantamab subcutaneously along with lazertinib may improve outcomes in EGFR-mutated advanced non-small cell lung cancer. Results from the PALOMA-3 trial showed that this approach not only reduces administration times but also led to a significant improvement in overall survival. Patients found the subcutaneous administration more convenient, with reduced infusion-related reactions. The efficacy and safety of the subcutaneous formulation were comparable to the intravenous version, but with shorter administration times and improved overall survival rates. This study represents a significant advance in delivering effective cancer therapies with reduced treatment burden.
Source link
Improved Outcomes With Subcutaneous Amivantamab in NSCLC