Key takeaways:
- A coformulation of vibostolimab and pembrolizumab was being studied for the adjuvant treatment of resected high-risk melanoma.
- The trial was stopped due to many patient discontinuations and adverse events.
Merck has discontinued the vibostolimab and pembrolizumab coformulation arm of its phase 3 trial for the adjuvant treatment of patients with resected high-risk melanoma, the company announced in a press release.
Pembrolizumab (Keytruda) is approved in the U.S. for the treatment of patients with unresectable or metastatic melanoma and for the adjuvant treatment of adult and pediatric patients with stage IIB, IIC or III melanoma following complete resection. Vibostolimab is Merck’s investigational anti-TIGIT antibody.

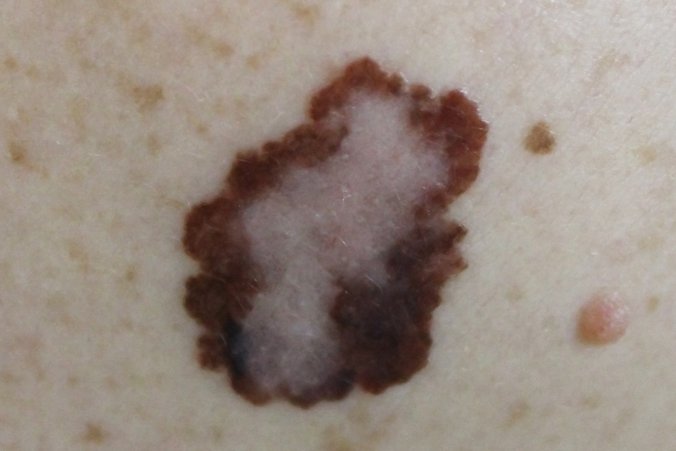
Merck has discontinued the vibostolimab and pembrolizumab coformulation arm of its phase 3 trial for the adjuvant treatment of patients with resected high-risk melanoma. Image: Adobe Stock.
Merck’s phase 3 study, KeyVibe-010, was investigating the coformulation of vibostolimab and pembrolizumab vs. Keytruda alone as an adjuvant treatment for patients with resected high-risk melanoma.
However, a higher rate of discontinuation in the coformulation arm vs. the Keytruda-only arm, mainly due to immune-mediated adverse events, has made it highly unlikely that recurrence-free survival, the study’s primary endpoint, can be achieved.
Based on an independent data monitoring committee recommendation, Merck has unblinded the study and recommended that patients receiving the coformulation be offered Keytruda monotherapy.
“Through our clinical development program, we continue to ask the tough questions in an effort to fully explore the potential of novel coformulations and combinations that build on the foundation of Keytruda,” Marjorie Green, MD, senior vice president and head of oncology, global clinical development at Merck Research Laboratories, said in the release. “We are grateful to the patients and investigators for their participation and will leverage insights from this trial as we rapidly advance our diverse pipeline of novel mechanisms.”
According to the press release, data analysis from KeyVibe-010 is still ongoing and will be shared with the scientific community and regulatory agencies in the future.