The US Food and Drug Administration (FDA) has announced that Tandem Diabetes Care, Inc has issued a recall regarding version 2.7 of the Apple iOS t:connect mobile app that is used in conjunction with the t:slim X2 insulin pump, which uses Control-IQ technology.
The recall, specifically referred to as a correction and not a product removal, was prompted by defective software in the app that can lead to the insulin pump’s battery draining, causing it to possibly shut down sooner than expected, suspending insulin delivery.
The action, a Class I recall, is the most serious type of FDA recall, indicating that “use of these devices may cause serious injuries or death,” the FDA reports.
As of April 15, 2024, the defect had led to 224 reported injuries but no reports of death.
On March 26, 2024, customers affected by the recall were sent an Urgent Medical Device Correction notice by Tandem Diabetes Care, Inc, requesting that they update their mobile app to version 2.7.1 or later, which is available through the Apple App Store.
Instructions for updating the app are included in the letter and detailed in the FDA recall notice.
The recall affects people with diabetes who may use the t:slim X2 Insulin Pump Mobile App version 2.7 on the Apple iOS platform as well as healthcare providers who may provide care using the technology.
For mobile apps that have already been updated to version 2.7.1 or later, no further update action is required.
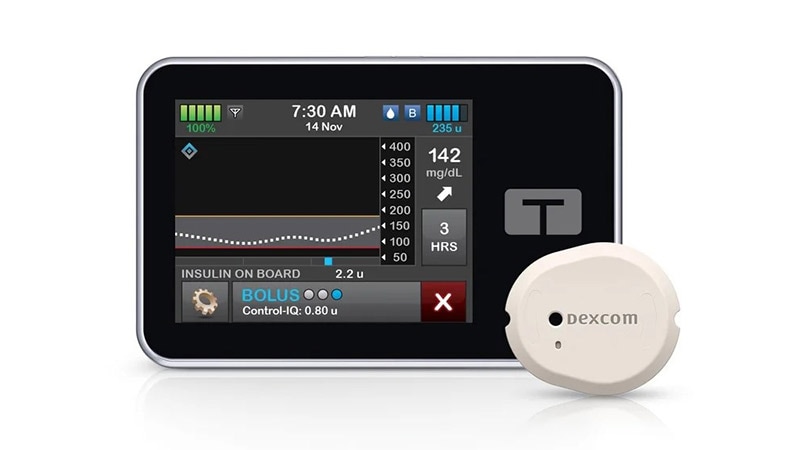
The t:slim X2 insulin pump is designed to deliver insulin subcutaneously at set and variable rates for the management of diabetes among people requiring insulin.
The t:connect mobile app works with the insulin pump with the Control-Q technology to allow viewing of pump information and limited control through smartphone and software technologies.
Due to an issue with the device’s software, the mobile app is specifically at risk of crashing and then being automatically relaunched by the iOS operating system. When this cycle intermittently repeats, excessive Bluetooth communication occurs, which potentially leads to the draining of the insulin pump battery and the subsequent shut-down of the pump.
When the delivery of insulin is suspended, the underdelivery of insulin poses the risk for hyperglycemia or even diabetic ketoacidosis, a potentially life-threatening condition. Though the pumps can continue to be used, patients should pay attention to all system alerts and alarms, the FDA cautions.
It is important to “monitor pump battery level closely to ensure the pump is at or near full charge before going to sleep to help prevent pump shutdown,” and users should “Begin charging device after the first low battery alert.” the FDA states.
“Begin charging device after the first low battery alert.”
In addition, users should always make sure to carry backup supplies for insulin delivery in case of the insulin pump’s failure.