RNA granules, sites for the storage, transport, and regulation of RNA molecules within cells, are transported along axons and then translated locally, far from the cell body. Recent studies suggest that these granules can “hitch a ride” from lysosome-related vesicles, membrane-bound structures in cells that share common characteristics with lysosomes.
Researchers at the National Institutes of Health recently set out to further investigate the transport of RNA granules alongside lysosomal vesicles, using a technique that prevents the transports of these vesicles.
Their findings, published in Nature Neuroscience, confirmed that a large cohort of RNA messengers are transported into the axon in conjunction with lysosomal vesicles, enabling local translation away from the soma or cell body (i.e., the primary part of a neuron).
“In previous research, our lab identified a multi-subunit complex named BORC, which links lysosomes to kinesin motors, enabling their movement along microtubule tracks toward the cell periphery,” Juan Bonifacino, co-author of the paper, told Medical Xpress.
“Consistent with this role, mutations in BORC subunits in both mice and humans, hinder the transport of lysosomal vesicles from the neuronal body to the axon. This disruption leads to axonal dystrophy and degeneration, ultimately resulting in severe neurological impairments.”
To carry out their experiments, Bonifacino and his collaborators leveraged the ability to halt the transport of lysosomal vesicles into the axons, by knocking out subunits of the BLOC-one related complex (BORC) uncovered in their previous studies. This method ultimately allowed them to explore the mechanism through which the lack of lysosomal vesicle transport compromises axonal health.
“We were particularly intrigued by the possibility that BORC-subunit KO prevented the axonal transport of not only lysosomal vesicles but also RNA granules that normally hitchhike on those vesicles, as shown by the labs of our colleagues Michael Ward (NINDS, NIH) and Jennifer Lippincott-Schwartz (HHMI-Janelia),” Bonifacino said.
To test the hypothesis that the KO of BORC subunits indirectly impedes the transport of some mRNAs into the axons, the researchers used a variety of advanced experimental techniques. First they used human neurons derived from induced pluripotent stem cells (iPSCs), which are easy to manipulate in laboratory settings, to silence or induce the expression of specific genes.
They then used a new microfluidic device developed at the National Institutes of Health, which can be used to isolate enough amounts of axonal material to perform RNA sequencing (RNA-Seq) and biochemical analyses. Finally, they monitored the transport of specific fluorescently labeled mRNAs using live imaging techniques, and used various experimental methods to examine both the structure and function of mitochondria.
“Our experiments revealed that a large cohort of mRNAs are transported into the axon in conjunction with lysosomal vesicles, enabling local translation away from the soma,” Bonifacino explained. “Messenger RNAs encoding mitochondrial and ribosomal proteins are particularly abundant in this cohort.
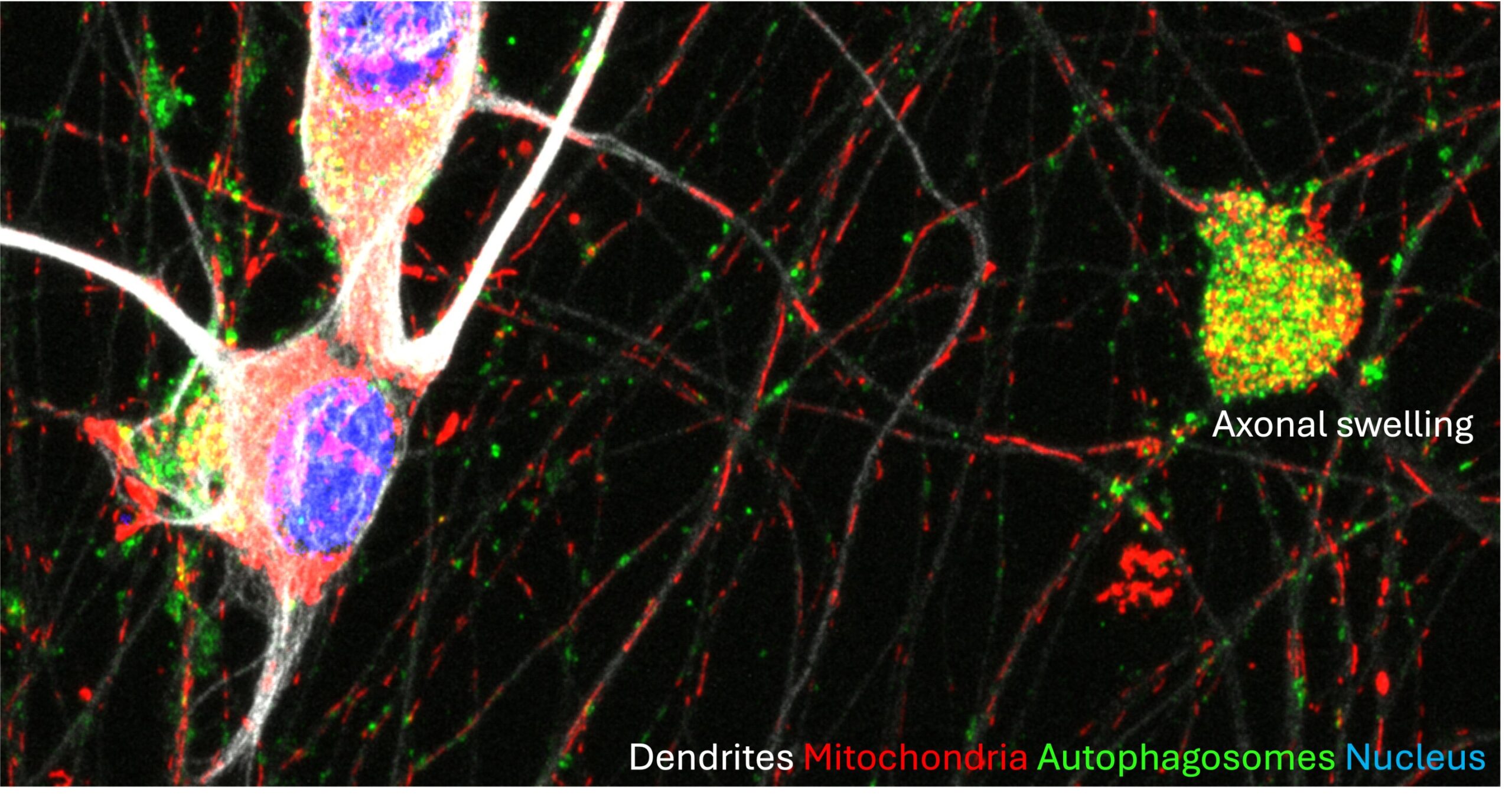
“Mitochondrial-related mRNAs play a crucial role in sustaining mitochondrial fitness, including maintaining the integrity of the oxidative phosphorylation system and membrane potential, and preventing excessive mitophagy. Consequently, this upkeep contributes to the preservation of axonal health. BORC-subunit knockout disrupts this process, leading to axonal dystrophy and degeneration.”
The recent study by Bonifacino and his collaborators demonstrated that the transport of mRNA on lysosome-related vesicles contributes to the maintenance of axonal homeostasis and compromising this transport causes axonal degeneration.
The newly uncovered mechanism of axonal degeneration in BORC-subunit-KO neurons could be related to the neurodegeneration associated with various disorders, including Alzheimer’s Disease and Parkinson’s Disease, which have both been linked to impairments in mitochondrial oxidative phosphorylation at some stage of their progression.
“We now plan to continue our studies of how defects in the axonal transport of lysosomal vesicles and mRNAs contribute to the pathogenesis of various developmental and neurodegenerative disorders,” Bonifacino added. “In particular, the ability to isolate pure axons using our microfluidic devices will enable more comprehensive analyses of axonal composition and function.”
More information:
Raffaella De Pace et al, Messenger RNA transport on lysosomal vesicles maintains axonal mitochondrial homeostasis and prevents axonal degeneration, Nature Neuroscience (2024). DOI: 10.1038/s41593-024-01619-1
© 2024 Science X Network
Citation:
Study finds that the transport of mRNAs into axons along with lysosomal vesicles prevents axon degeneration (2024, May 6)
retrieved 6 May 2024
from https://medicalxpress.com/news/2024-05-mrnas-axons-lysosomal-vesicles-axon.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.