Key takeaways:
- The adjusted difference of PASI from baseline at week 12 was –0.6%.
- PGA, DLQI and treatment-emergent adverse events were also comparable.
SB17 was clinically biosimilar to ustekinumab for the treatment of moderate to severe psoriasis, according to a study.
“Biologics have a unique role in treatment of chronic inflammatory diseases, including psoriasis, however high cost has been a barrier to treatment access,” Steven R. Feldman, MD, PhD, professor of dermatology, pathology and social sciences and health policy at Wake Forest University School of Medicine, and colleagues wrote. “The development of biosimilars … is intended to improve the quality of patient care by reducing barriers to access, with realizations of cost-savings, in both the U.S. and Europe.”

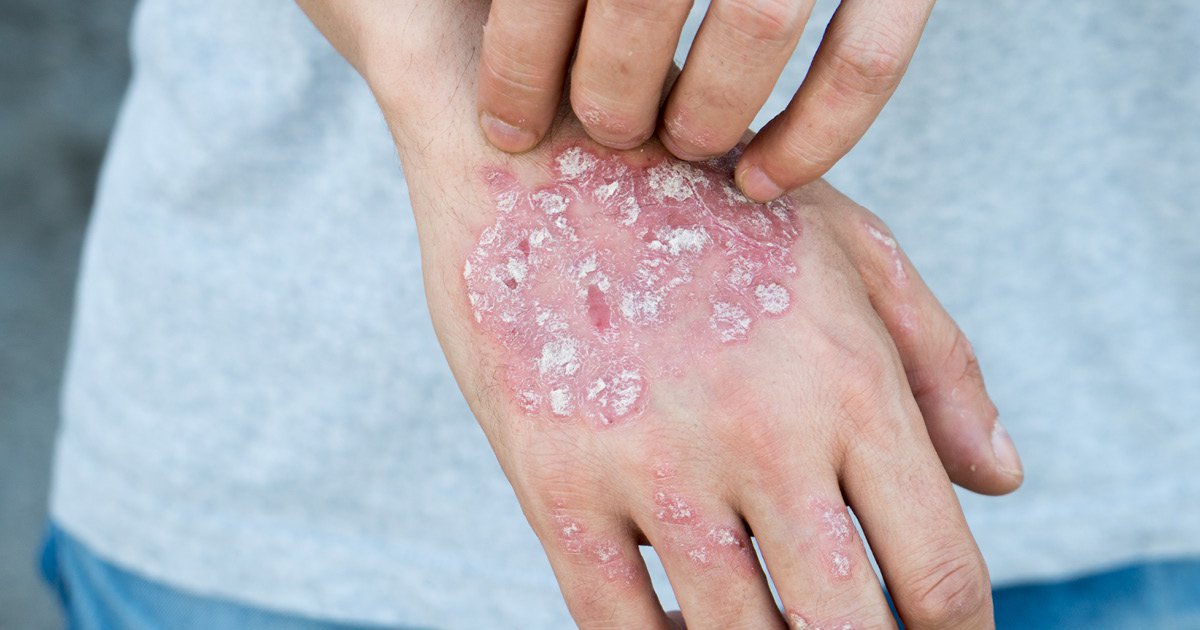
SB17 was clinically biosimilar to ustekinumab for the treatment of moderate to severe psoriasis. Image: Adobe Stock.
SB17 (Samsung Bioepis Co.) is a proposed biosimilar of the reference drug ustekinumab. While SB17 was physicochemically similar and pharmacokinetically bioequivalent in a phase 1 study, the current study set out to assess whether the drug has similar efficacy, safety, pharmacokinetics and immunogenicity to ustekinumab up to week 28.

Steven R. Feldman
In the study, 503 adult patients with moderate to severe psoriasis were randomly assigned to receive 45 mg of SB17 (n = 249) or ustekinumab (n = 254) subcutaneously at week 0, 2 and then every 12 weeks up to week 28.
Results showed that the adjusted difference of PASI from baseline at week 12 was –0.6% (95% CI, –3.78 to 2.579), landing the biosimilar within the equivalent margin of ustekinumab. Similarly, the PGA and DLQI were also comparable.
The rate of treatment-emergent adverse events was nearly identical, occurring in 48.2% of the SB17-treated patients and 48.8% of the ustekinumab-treated patients.
“The relative difference of [anti-drug antibodies] incidence may be due to differences in cell lines that produce the monoclonal antibodies,” the researchers hypothesized.
Overall, the researchers concluded the SB17 has similar efficacy and comparable safety and pharmacokinetics to reference ustekinumab, as well as lower immunogenicity up to week 28 in patients with moderate to severe psoriasis.