A team of researchers has uncovered the identity of off-target cells responsible for abnormal outgrowth and devised a method to eliminate them from iPS cell-derived pancreatic tissues in development for cell therapy against type 1 diabetes.
The study is published in the Proceedings of the National Academy of Sciences and was led by Junior Associate Professor Taro Toyoda (Department of Life Science Frontiers, CiRA) and included researchers from CiRA, Takeda Pharmaceutical Co. Ltd., and Axcelead Drug Discovery Partners, Inc.
iPS cells, one of two types of pluripotent stem cells (PSCs), are considered a valuable cell source for generating various cells and tissues for cell-based therapies, including type 1 diabetes treatment. The potential of iPS cells to become nearly any cell type in the body also creates concerns about possible risks of off-target cells and tumorigenesis.
Many reports have described unintended graft growth following the implantation of PSC-derived pancreatic progenitors or endocrine cells into mice, reinforcing concerns about their safety. Therefore, it is necessary to eliminate off-target cells and control the differentiation and proliferation of iPS cell-derived cell products before they can be used for clinical applications.
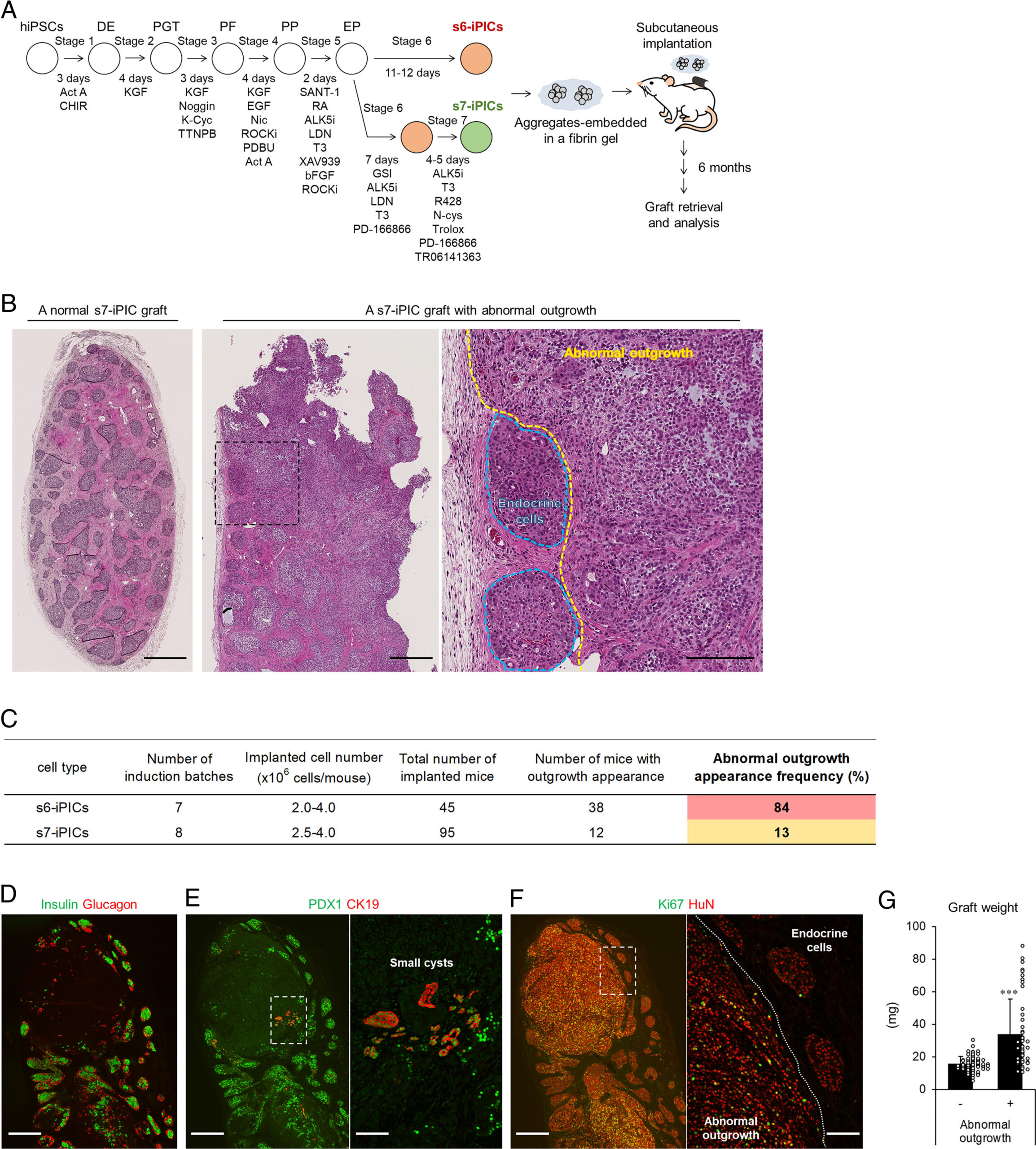
The researchers have been refining an induction protocol to direct the differentiation of induced PSC-derived pancreatic islet cells (iPICs) from iPS cells. In the current study, the researchers continued this ongoing effort and implanted iPICs generated using a recently established six- or seven-step induction protocol (s6- or s7-iPIC grafts) into mice to assess their long-term safety.
Whereas most s7-iPICs did not show any abnormalities, many s6-iPIC grafts resulted in large cysts or unexpected outgrowth, suspected to arise from residual nonendocrine progenitor cells eliminated by PD-166866 treatment included in the s7-PIC induction protocol.
Through histological and single-cell RNA sequencing (scRNA-seq) analysis, the researchers contributed this unwanted outgrowth to proliferative mesenchymal stem cell (MSC)- and smooth muscle cell (SMC)-like cells (PMSCs).
The team also revealed that PMSCs are not specific to their iPIC induction method, as the re-examination of scRNA-seq data from an independent study examining in vivo grafts of PSC-derived islet-like cells also revealed the presence of a cell population displaying PMSC-specific markers. Furthermore, they found that PMSCs, which appear to arise only after transplantation, exhibited uterine characteristics.
Having identified these unwanted cells, the research team next searched for ways to eliminate these PMSCs to prevent the initially observed abnormal outgrowth. As mentioned, because PMSCs appear to arise only following implantation and screening for means for eliminating them under in vivo conditions would require a tremendous amount of time and experimental animals, the researchers attempted to simulate post-implantation in vivo conditions to induce the appearance of PMSCs in vitro.
To this end, they determined that a combination of extended culturing time and epidermal growth factor (EGF)-supplemented medium leads to the in vitro generation of a cell population similar to PMSCs after transplantation.
Using this modified induction protocol, the research team designed ways to eliminate the undesirable PMSCs. Knowing that s7-iPICs do not yield as many PMSCs after implantation compared to s6-iPICs, they tested the ability of R428, N-acetylcysteine, and Trolox, factors s7- but not s6-iPICs are treated with, to determine whether they are responsible for limiting PMSC generation. While N-acetylcysteine and Trolox had no effects, R428, an AXL inhibitor, significantly reduced putative PMSCs from s6-PICs.
In addition, because PMSCs exhibit uterine characteristics, the researchers also found lenvatinib (a multikinase inhibitor indicated for endometrial carcinoma) to possess similar efficacy as R428 in preventing PMSC generation. However, they reasoned that kinase-independent mechanisms are likely responsible for PMSC generation because some PMSCs can still arise even though several kinase inhibitors are used during s7-iPIC differentiation.
Instead, since platinum-based compounds, such as cisplatin, and taxanes, like docetaxel and paclitaxel, are commonly used as first-line chemotherapies against endometrial carcinomas, the research team also investigated whether they could be effective for eliminating PMSCs. Indeed, cisplatin and docetaxel effectively prevented PMSC generation without any observable effects on the desired cell types that mature into β- and α-cells following implantation.
Finally, to demonstrate the effectiveness of docetaxel treatment as a viable means to eliminate PMSCs from s7-PICs after in vivo transplantation, the researchers implanted s7-iPICs with or without docetaxel treatment into drug-induced type 1 diabetes model mice. As expected, s7-iPICs with and without docetaxel treatment effectively restored insulin production and blood glucose regulation.
Remarkably, no PMSCs or cysts were detected in either case. To further illustrate the effects of docetaxel on removing unwanted cell types, the research team also implanted s6-iPICs, known to be more problematic regarding cyst formation and PMSC generation, with or without docetaxel treatment.
Similarly, PMSCs and large cysts were completely absent when s6-iPICs were treated with docetaxel, thus demonstrating the ability of docetaxel to eliminate undesirable cells before transplantation.
Through this study, the research team not only identified the cells responsible for the abnormal outgrowth of transplanted iPS cell-derived pancreatic tissues, but they also identified an effective means to eliminate them by including an additional docetaxel treatment step in the induction protocol.
Therefore, with their latest findings, the safety profile of iPS cell-based therapies for type 1 diabetes is one crucial step closer to ensuring patient safety.
More information:
Hideyuki Hiyoshi et al, Identification and removal of unexpected proliferative off-target cells emerging after iPSC-derived pancreatic islet cell implantation, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2320883121
Citation:
Improving the safety of iPS cell-derived pancreatic islets by eliminating unwanted cells (2024, May 13)
retrieved 13 May 2024
from https://medicalxpress.com/news/2024-05-safety-ips-cell-derived-pancreatic.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.