Key takeaways:
- TDM-180935 is a small molecule Janus kinase/tyrosine kinase 2 inhibitor for the topical treatment of atopic dermatitis.
- The phase 2a trial will span 8 weeks and evaluate the safety and efficacy of the drug.
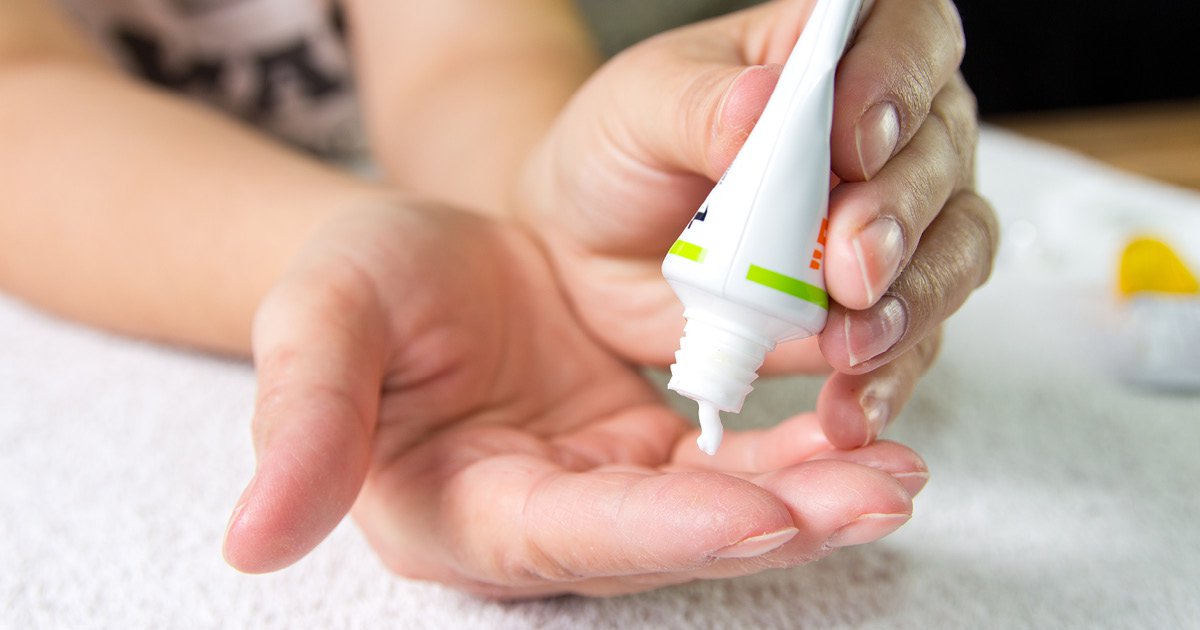
Technoderma Medicines has dosed its first patients in its phase 2a clinical trial of topical TDM-180935 ointment for the treatment of atopic dermatitis, the company announced in a press release.
The randomized, vehicle-controlled, parallel group comparison study, which falls into the company’s AD program, includes 8 weeks dosing of two different active formulation strengths and placebo. The study will evaluate the safety and efficacy of topical TDM-180935 as well as the drug’s pharmacokinetics, which will be analyzed in an open-label sub-study.

Technoderma Medicines has dosed its first patients in its phase 2a clinical trial of topical TDM-180935 ointment for the treatment of atopic dermatitis. Image: Adobe Stock.
“We are pleased with the excellent toleration and minimal systemic absorption already exhibited by TDM-180935 ointment in phase 1 development and now look forward to assessing its preliminary efficacy in atopic dermatitis,” Zengquan Wang, PhD, CEO of Technoderma Medicines, said in the release. “The advancement of this second program in our portfolio into phase 2 development validates our capabilities in developing multiple pipeline products for treatment of dermatologic diseases.”
TDM-180935 is a small molecule drug candidate for the topical treatment of AD. According to the release, the drug is a potent Janus kinase 1/tyrosine kinase 2 inhibitor, which positions it to possibly offer more efficacious results than other topical treatments