A new study looking at the distribution of receptors that flu viruses can attach to in different cow tissues may help to explain the pattern of illness being seen in the outbreak of H5N1 bird flu in U.S. dairy cattle — and also is sparking debate about the implications of the cow outbreak for human disease.
The study, a preprint that hasn’t yet been through peer review by a scientific journal, found that tissue from the mammary gland contains abundant receptors of the kind to which avian flu viruses like H5N1 can attach. But brain and respiratory tract tissues contained far fewer of this type of receptor.
That fits with the description of how this virus is behaving in cows, with clear signs of infection in the udders, but little evidence of respiratory tract infections and no reports to date of cows experiencing neurological symptoms. This virus has been seen to attack the brains of a number of other mammalian species it can infect, including cats, which is why consideration of whether cows’ brains are also susceptible is important.
The work was done by scientists at the University of Copenhagen, Denmark’s Statens Serum Institut, and St. Jude Children’s Research Hospital in Memphis, Tenn.
The distribution of the receptor types — some of which avian flu viruses can attach to, others to which human flu viruses attach — raises concerns that cows could potentially give rise to a version of H5N1 or another novel flu virus that is able to transmit efficiently to and among people, the authors suggested.
“These results provide a mechanistic rationale for the high levels of H5N1 virus reported in infected bovine milk and show cattle have the potential to act as a mixing vessel for novel [influenza virus] generation,” they wrote.
Pigs, which can be infected with both bird flu and human flu viruses, have traditionally been referred to as mixing vessels for new viruses that could pose a pandemic threat. That’s because when an animal is co-infected with different flu viruses, the pathogens can swap genes, a process called reassortment. That phenomenon could give rise to new virus strains with enough human genes to be able to easily infect people, but also enough bird flu genes to be foreign enough to human immune systems that they could pose a serious risk to human health.
Cows have not been thought to pose such a risk. Though it’s known they can be infected with flu viruses, influenza hasn’t been seen to be a major issue for cows. And until this outbreak, cows have not played a role in the H5N1 story.
This virus, first seen in China in 1996, is primarily one that infects wild birds and domestic poultry, though it has been shown to be able to infect numerous mammalian species over the nearly three decades that have followed. In that time, nearly 900 human infections have been detected. Of those people, roughly half have died.
In the current U.S. outbreak, one human case has been detected, a dairy farm worker in Texas whose sole symptom was conjunctivitis. It’s widely believed there may have been other cases, but farmers have in many cases been unwilling to cooperate with health authorities trying to investigate the outbreak and many of the workers have been wary of cooperating as well.
Some scientists not involved in the research described the paper as interesting, but questioned whether one could conclude cows pose a mixing vessel risk based on these findings.
Malik Peiris, chair of virology in the University of Hong Kong’s School of Public Health, told STAT in an email that previous research has shown that cows are not highly susceptible to human flu viruses. Furthermore, it would be hard to see how a human flu virus could reach a cow’s udder, which contains receptors for both avian and human flu viruses. “Thus, I do not think the risk of human flu-H5N1 reassortment in cows is likely.”
Angela Rasmussen, a virologist at the Vaccine and Infectious Disease Organization at the University of Saskatchewan, in Saskatoon, Canada, agreed, noting that knowing which tissues a flu virus can attach to only tells part of the story. There are other factors, she said, that determine whether or not a virus can replicate in the cells it attaches to, triggering infection.
That said, Rasmussen, who studies animal diseases that can jump into people, is still worried about H5N1 in cows. To date, H5N1 infections have been confirmed in 36 herds in nine states.
“I’m more concerned about cow H5 evolving toward human-to-human transmission by jumping between different hosts (bird to cow to human, cow to bird to cat, cow to cat) as well as cow to cow. This selects for viruses that can infect, replicate efficiently, and shed enough to transmit onward in many different hosts, and increases the odds that it could do so in a human one,” she wrote in an email.
“These data are confirmation to me that it is essential to get the cow outbreak under control, because there are many possibilities for a more transmissible human virus to emerge from it.”
The Danish researchers took samples of stored tissues — brain, respiratory tract, and mammary gland — from a small number of cows and a calf. Some of the cows were dairy cows; others were beef cattle. They stained the tissue to see what receptors they had, whether they were for human or avian flu viruses, and among the latter whether they were the type best adapted for viruses that circulate among chickens or those that circulate in wild ducks. A spillover of virus from wild birds is believed to have started this outbreak in cows.
They found few receptors in the brain tissue they examined. “What we found was that the influenza receptors were expressed in the neurons, but in a very low amount. So we also speculate that this could be the reason why there have not been found any neurological signs” among infected cattle, said first author Charlotte Kristensen. Kristensen is defending her thesis this week and is set to begin postdoctoral research at the university, in the department of veterinary and animal sciences.
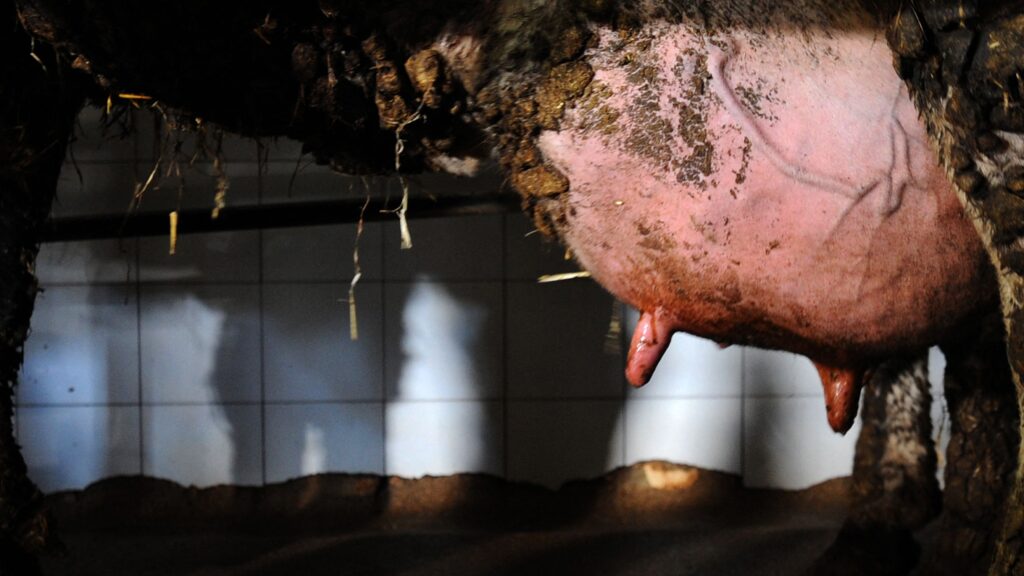
A surprising finding was a lack of receptors in the ducts of the mammary glands, which the authors said argues against the notion that cows are becoming infected by what they call an “ascending infection.” The leading hypothesis for how this infection is spreading among cows is that when an infected cow enters a herd, small amounts of its H5N1-contaminated milk can remain on milking equipment, coming in contact with the teats of uninfected cows, who then become infected.
Peiris, while agreeing the receptor pattern in the mammary ducts was surprising, said he still believes this route of infection is most likely, noting that there are reports that some cows only experience infection in some — not all — quarters of their udders. “There is no other way to explain how one segment of the mammary gland is infected while others are unaffected,” Peiris said.
Rasmussen agreed. “These results are interesting and good to know. But I don’t think they are sufficient evidence to rule out contaminated milking equipment or any other hypothetical transmission route. Especially respiratory,” she wrote.