SAN DIEGO — Compared with medical oncologists, dermatologists were more likely to correctly classify and grade dermatologic adverse events from cancer therapies, results from a multicenter survey showed.
“New cancer therapies have brought a diversity of treatment-related dermatologic adverse events (dAEs) beyond those experienced with conventional chemotherapy, which has demanded an evolving assessment of toxicities,” researchers led by Nicole R. LeBoeuf, MD, MPH, of the Department of Dermatology at Brigham and Women’s Hospital and the Center for Cutaneous Oncology at the Dana-Farber Brigham Cancer Center, Boston, wrote in a poster presented at the American Academy of Dermatology (AAD) 2024 Annual Meeting.
The authors noted that “Version 5.0 of the Common Terminology Criteria for Adverse Events (CTCAE v5.0)” serves as the current, broadly accepted criteria for classification and grading during routine medical care and clinical trials. But despite extensive utilization of CTCAE, there is little data regarding its application.”
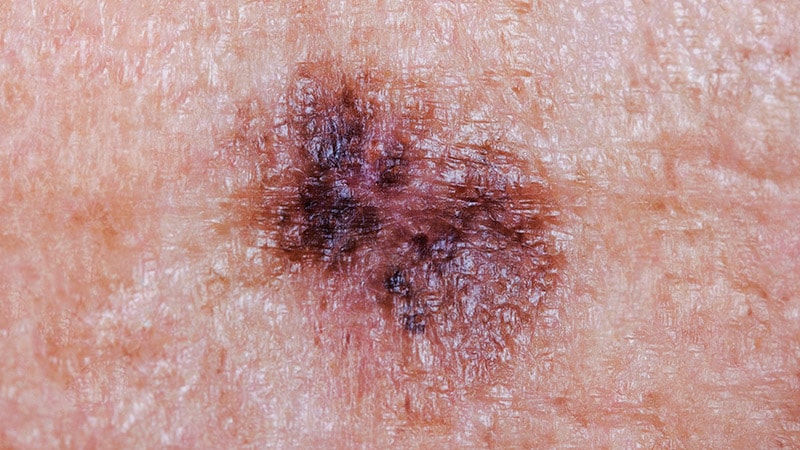
To evaluate how CTCAE is being used in clinical practice, they sent a four-case survey of dAEs to 81 dermatologists and 182 medical oncologists at six US-based academic institutions. For three of the cases, respondents were asked to classify and grade morbilliform, psoriasiform, and papulopustular rashes based on a review of photographs and text descriptions. For the fourth case, respondents were asked to grade a dAE using only a clinic note text description. The researchers used chi-square tests in R software to compare survey responses.
Compared with medical oncologists, dermatologists were significantly more likely to provide correct responses in characterizing morbilliform and psoriasiform eruptions. “As low as 12%” of medical oncologists were correct, and “as low as 87%” of dermatologists were correct (P < .001). Similarly, dermatologists were significantly more likely to grade the psoriasiform, papulopustular, and written cases correctly compared with medical oncologists (P < .001 for all associations).
“These cases demonstrated poor concordance of classification and grading between specialties and across medical oncology,” the authors concluded in their poster, noting that 87% of medical oncologists were interested in additional educational tools on dAEs. “With correct classification as low as 12%, medical oncologists may have more difficulty delivering appropriate, toxicity-specific therapy and may consider banal eruptions dangerous.”
Poor concordance of grading among the two groups of clinicians “raises the question of whether CTCAE v5.0 is an appropriate determinant for patient continuation on therapy or in trials,” they added. “As anticancer therapy becomes more complex — with new toxicities from novel agents and combinations — we must ensure we have a grading system that is valid across investigators and does not harm patients by instituting unnecessary treatment stops.”
Future studies, they said, “can explore what interventions beyond involvement of dermatologists improve classification and grading in practice.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC, who was asked to comment on the study, noted that with the continued expansion and introduction of new targeted and immunotherapies in the oncology space, “you can be sure we will continue to appreciate the importance and value of the field of supportive oncodermatology, as hair, skin, and nails are almost guaranteed collateral damage in this story.”
“Ensuring early identification and consistent grading severity is not only important for the plethora of patients who are currently developing the litany of cutaneous adverse events but to evaluate potential mitigation strategies and even push along countermeasures down the FDA approval pathway,” Friedman said. In this study, the investigators demonstrated that work “is sorely needed, not just in dermatology but even more so for our colleagues across the aisle. A central tenet of supportive oncodermatology must also be education for all stakeholders, and the good news is our oncology partners will welcome it.”
LeBoeuf disclosed that she is a consultant to and has received honoraria from Bayer, Seattle Genetics, Sanofi, Silverback, Fortress Biotech, and Synox Therapeutics outside the submitted work. No other authors reported having financial disclosures.
Friedman directs the supportive oncodermatology program at GW that received independent funding from La Roche-Posay.