Unlocking Agentic RL Training for GPT-OSS: A Practical Retrospective
Summarize this content to 100 words: Agentic reinforcement learning (RL) extends traditional LLM training by optimizing not just a single-turn response, but an entire decision-making process learned through direct interaction…
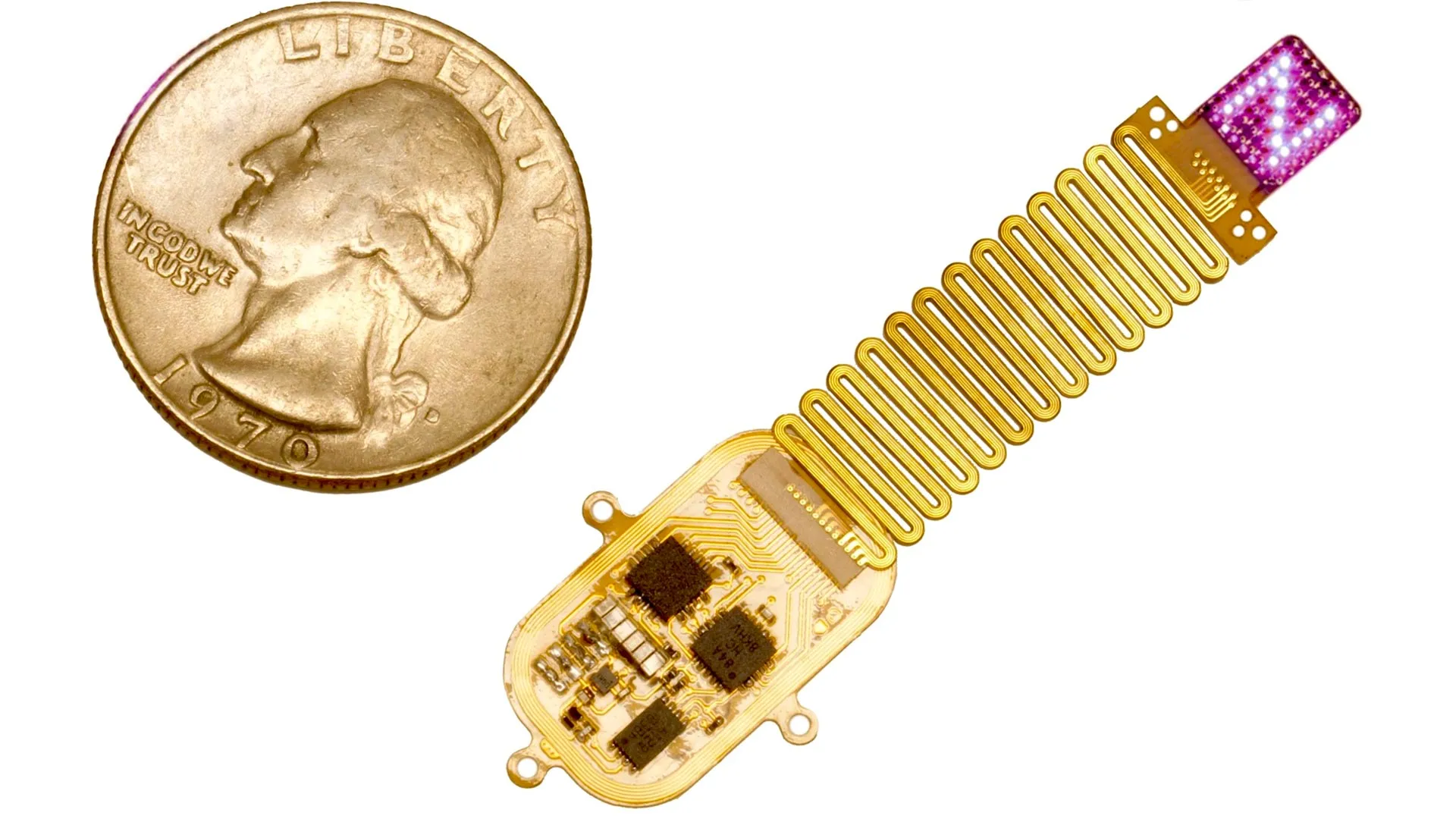
This tiny implant sends secret messages to the brain
Summarize this content to 100 words: In a major step forward for neurobiology and bioelectronics, scientists at Northwestern University have created a wireless device that uses light to transmit information…
NotebookLM Brings Cinematic Video Overviews
Summarize this content to 100 words: Whenever I am to suggest a new AI tool to someone who is just starting out, there is one name I know can bring…
Agent Observability and Evaluation: A 2026 Developer’s Guide to Building Reliable AI Agents
Summarize this content to 100 words: Last Updated on March 4, 2026 by Editorial Team Author(s): Divy Yadav Originally published on Towards AI. Why building agents without this layer is…
Sarvam Edge: A Beginner’s Guide to On-Device AI for India
Summarize this content to 100 words: Suppose there is a smart computer in your cell phone. It responds instantly, knows your language, and is completely functional even without the internet.…
I Built an Ontology Firewall for Microsoft Copilot in 48 Hours — Here’s the Production Code
Summarize this content to 100 words: Last Updated on March 4, 2026 by Editorial Team Author(s): Pankaj Kumar Originally published on Towards AI. Most Copilot deployments are one bad agent…
Remote Health: From Crisis to Prevention
Summarize this content to 100 words: By Rama Kolli, CIO, Blue Cross and Blue Shield of Nebraska A tale of the not-too-distant future- Joe is 76 years old and lives…
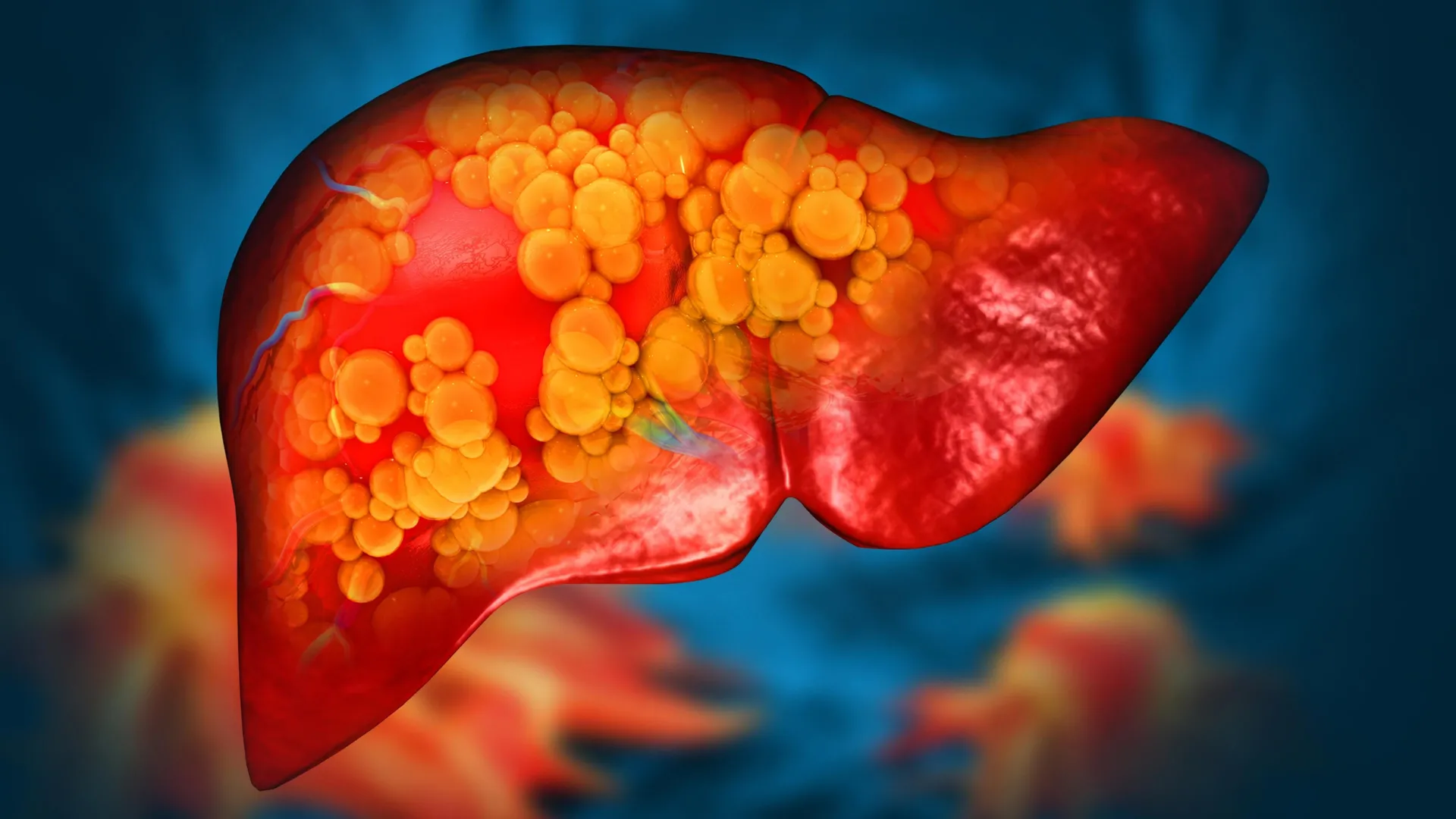
Mayo Clinic discovers rare gene mutation that causes fatty liver disease
Summarize this content to 100 words: Scientists at Mayo Clinic's Center for Individualized Medicine have identified a rare genetic variant that can directly cause metabolic dysfunction-associated steatotic liver disease, formerly…
Jack Dorsey Is Ready to Explain the Block Layoffs
Summarize this content to 100 words: In an exclusive interview with WIRED, Block’s cofounder and CEO says he axed 40 percent of his workforce so that he can rebuild the…
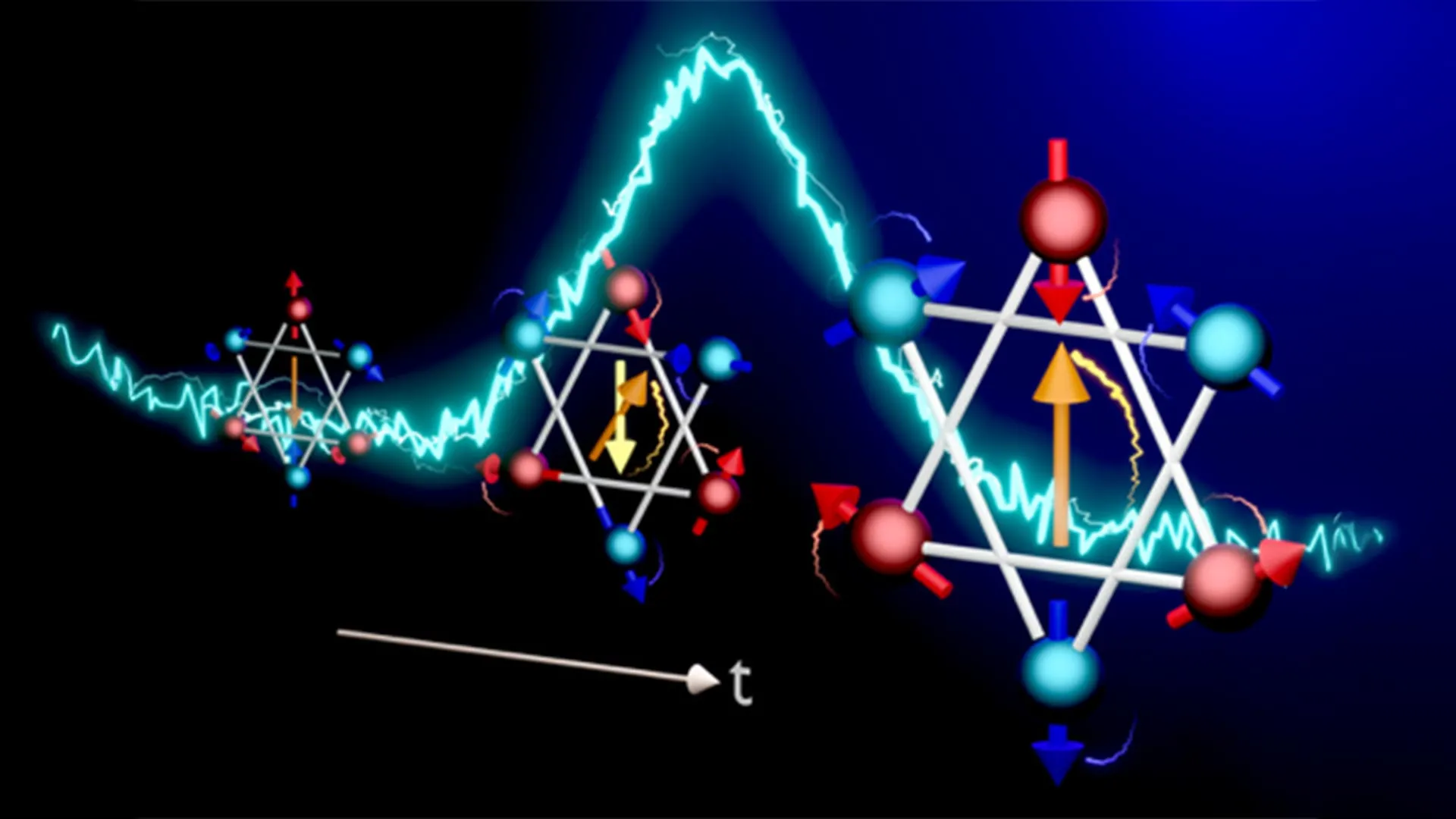
Scientists capture a magnetic flip in 140 trillionths of a second
Summarize this content to 100 words: A team led by Ryo Shimano at the University of Tokyo has directly observed how electron spins flip inside an antiferromagnet, a material in…